Next generation drug screening platform for precision oncology services
Where are the challenges in setting up a drug screening platform? What benefits can be expected from a new approach to personalized medicine? CBmed builds up on experiences won by Asian partners and forms a consortium to establish a new kind of drug screening technology by integrating a new developed metabolomics platform.
Drug screening itself might not be a new technology – but the clinical demand remains high, particularly the need for a faster and more precise approach. Drug screening in advanced cancer generally aims to find a solution for a fundamental medical problem: Which drug is the most effective therapy for the patient? Especially in the treatment of cancer patients with an advanced disease, it is likely that therapies fail to be effective. Besides a long and arduous task for patients, the response rate is comparatively low at around 20% to 30%. And while patients are losing precious time, it is also a financial issue: Many drugs cost a considerable amount of money, and though the prescription of a therapy must be well-founded, failure cannot be ruled out. So while patients need the right drug at the right time with the right dosage, the pharmaceutical industry is highly interested in effective medication as well. Therefore, the need for a tool to predict the effectiveness of a cancer therapy is high. This is where the next generation drug screening platform for precision oncology services steps in.
Specialists working in the labs of CBmed in Graz (Austria).
Long term cell cultures vs. short term cultures
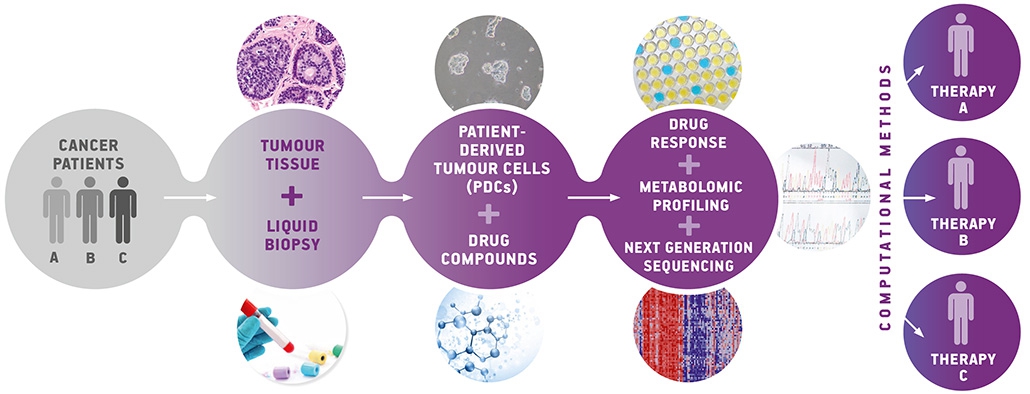
Basically, precision oncology aims to provide personalized treatment options by identifying, monitoring and targeting molecular aberrations of cancer cells in individual patients. In this regard, ex-vivo drug screening systems have the potential to improve clinical outcomes. Cancer drugs are commonly tested on long-term cultured cancer cell line models – a procedure which has led to a better understanding of cancer biology in general. But: long term cultured cell lines cannot represent an individual new patient from a clinic and they are biologically too far away to be informative for drug screening efforts. Patient derived tumor xenograft models (PDXs) overcome this problem by implanting tumor cells into immunodeficient or humanized mouse models. But it takes too much time until the drug response can be quantified and used to support therapeutic decisions for individual patients. For drug screening systems of tumor cells, viability assays are frequently used and sometimes correlated to a genetic profile to stratify patients. One of the pioneers in this field has been Professor Do-Hyun Nam and his team at the Samsung Medical Center in Seoul. Over the past 10 years, he developed a drug screening system with short term cultures of patient derived tumor cells (PDCs). Therein, efficacy profiles of various drugs are screened within just 3 weeks, using PDCs enriched with additional data, such as genomic analyses. One of the most crucial tasks is the development of the short term culture conditions – a delicate process since different tumor cells require different conditions to grow. Some of them, such as prostate or breast cancer, are very difficult to cultivate since they are heavily depending on hormones, which is hard to mimic in a cell culture. Other tumor cell cultures, such as colon cancer cells, are challenging because of the high risk of contamination caused by bacteria commonly residing in the gut tissue.
The workflow for the next generation drug screening platform.
A new approach – metabolomics
Now, measuring drug response and combining it with metabolomics opens new opportunities to gain a deeper insight into drug resistant profiles of tumor cells. This scientific project will kick off with all partners in October 2020 in Graz (Austria) and will focus on using PDCs from individual cancer patients. In a first step, the drug screening platform will be established at CBmed, based on the standardised viability- and genetic assays already published. Next, a novel mass spectrometry platform will be used to investigate the metabolomic profiles of PDCs before and after drug treatment. By combining these three data sets – viability, genetics, metabolomics – with the pharmacological readout of the drug screening, it is possible to receive a more complete picture of the molecular backgrounds of drug resistance or sensitivity. This so called “next generation drug screening platform” will require even more sophisticated levels of data analysis compared to the standard methodology. Nevertheless, the result will lead to an optimised drug screening platform which will support future treatment decisions.
A transcontinental partnership: Prof. Pierce Chow (Chief Medical Officer, AvataMed), Dr. Young Yun (CEO, Avatamed), in front Prof. Thomas Pieber (CSO, CBmed), Prof. Peter Schemmer (Med Uni Graz), Robert Lobnig (CFO, CBmed).
Solutions through strategic partnerships
A project like this requires strategic partnerships and cooperations, and as a research center, CBmed serves as an interface to several medical key-players. A link to Asia has been built up and deepened over the years, achieving a partnership consisting of Aimed Bio located in South Korea, AvataMed located in Singapore and the Japanese Shimadzu Corporation as one of the worldwide leading suppliers of analytical instruments. Besides these international companies, several scientific partners will be cooperating within the project. The Medical University of Graz (MU) will contribute expertise in setting up and realizing a prospective clinical pilot study, in standardized pre-analytical sample handling, expertise in genomic analysis and in the assessment of quality parameters for human tissue. The Medical University of Vienna (MUW) will be a second center for the prospective clinical pilot study. Finally, Joanneum Research Health (JR) will mainly offer expertise in the field of metabolomics, sample preparation and metabolomics data analysis. CBmed is bringing all data and technologies together to establish the next generation drug screening platform. This scientific project has now been set until 2022, and by then, the system should be established and optimized to a level that allows to take on the next step: a clinical study where a benefit of drug screening system shall be investigated for patient treatment.
The Authors
Priv.-Doz. Amin El-Heliebi, Ph.D., CBmed GmbH, Scientific Area Leader “Cancer”
Mag.a rer.nat. Barbara Prietl, Ph.D, CBmed GmbH, Head of Core Technologies